Taking your clinical surveillance and compliance programs to the next level
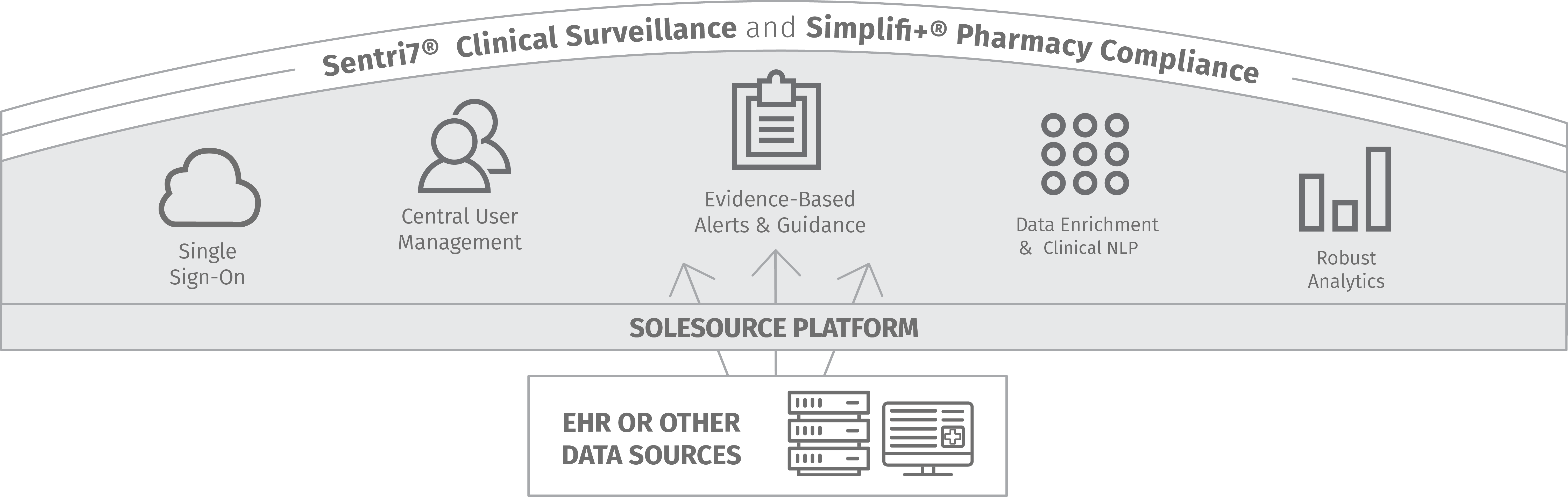
Wolters Kluwer's clinical surveillance and compliance applications are deployed on a single platform for easy integration with EHRs or into existing workflows while minimizing the impact on resource-strapped IT and clinical teams. Customers scale and standardize programs for maximum impact on patient safety, quality care measures, and financial performance.
Access our turnkey applications on the SoleSource platform
Sentri7® Clinical Surveillance and Simplifi+® Compliance applications help customers solve a wide range of healthcare challenges in departments and across facilities and health systems. One platform makes it easier to manage applications and end users and add on applications and locations as surveillance and compliance needs change.